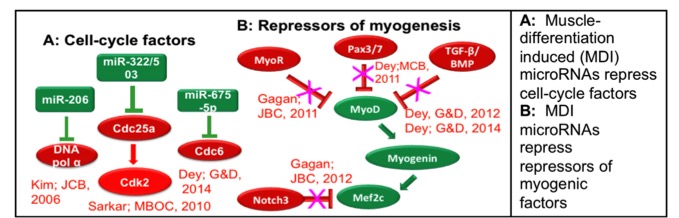
Non-coding RNAs
Half my lab studies how non-coding RNAs regulate differentiation and cancer. We identified many microRNAs that are induced during skeletal muscle differentiation and showed that they have specific roles in promoting cell-cycle quiescence and regulating myogenic transcription factors (Figure). In parallel studies in prostate cancer we identified the miR-99 family of microRNAs as tumor suppressors. Our work on differentiation and cancer progression has been moving towards the identification of long noncoding RNAs (lncRNAs) that are important for these processes. For example, we have identified lncRNAs H19. MUNC and ten others as being important for muscle differetiation, with significant effort on mechanisms by which these lncRNAs regulate cellular function. In parallel we have identified lncRNAs like DRAIC and APTR that are critical for cancer cell proliferation or cell migration/invasion.

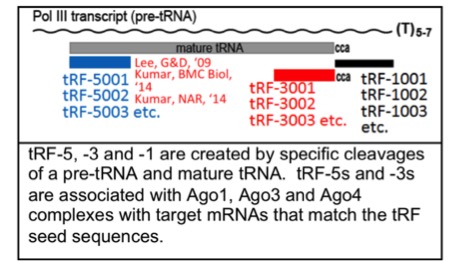
We have made the novel discovery that our cells are full of short RNAs (tRFs) that are derived from specific cleavage of tRNAs. These tRFs are a class of non-micro-short RNAs that enter into Ago complexes and use their seed sequences to target mRNAs. We have created the first tRF database to expedite the study of tRFs. We believe that tRFs and lncRNAs are both going to become significantly important in our studies of differentiation and cancer.

Relevant publications from the lab:
Przanowska RK, Weidmann CA, Saha S, Cichewicz MA, Jensen KN, Przanowski P, Irving PS, Janes KA, Guertin MJ, Weeks KM, Dutta A. Distinct MUNC lncRNA structural domains regulate transcription of different promyogenic factors. Cell Rep. 2022
Saha S, Kiran M, Kuscu C, Chatrath A, Wotton D, Mayo MW, Dutta A. Long noncoding RNA DRAIC inhibits prostate cancer progression by interacting with IKK to inhibit NF-kB activation. Cancer Res. 2020
Su Z, Wilson B, Kumar P, Dutta A. Noncanonical Roles of tRNAs: tRNA Fragments and Beyond. Annu Rev Genet. 2020
Przanowska RK, Weidmann CA, Saha S, Cichewicz MA, Jensen KN, Przanowski P, Irving PS, Janes KA, Guertin MJ, Weeks KM, Dutta A. Distinct MUNC lncRNA structural domains regulate transcription of different promyogenic factors. Cell Rep. 2022
Saha S, Kiran M, Kuscu C, Chatrath A, Wotton D, Mayo MW, Dutta A. Long noncoding RNA DRAIC inhibits prostate cancer progression by interacting with IKK to inhibit NF-kB activation. Cancer Res. 2020
Su Z, Wilson B, Kumar P, Dutta A. Noncanonical Roles of tRNAs: tRNA Fragments and Beyond. Annu Rev Genet. 2020